Your guide to blood-based breast cancer screening
The following educational resources are intended to help you become familiar with the clinical evidence supporting the efficacy and safety of Cantel™, a blood-based screening test for breast cancer
Download Brochure

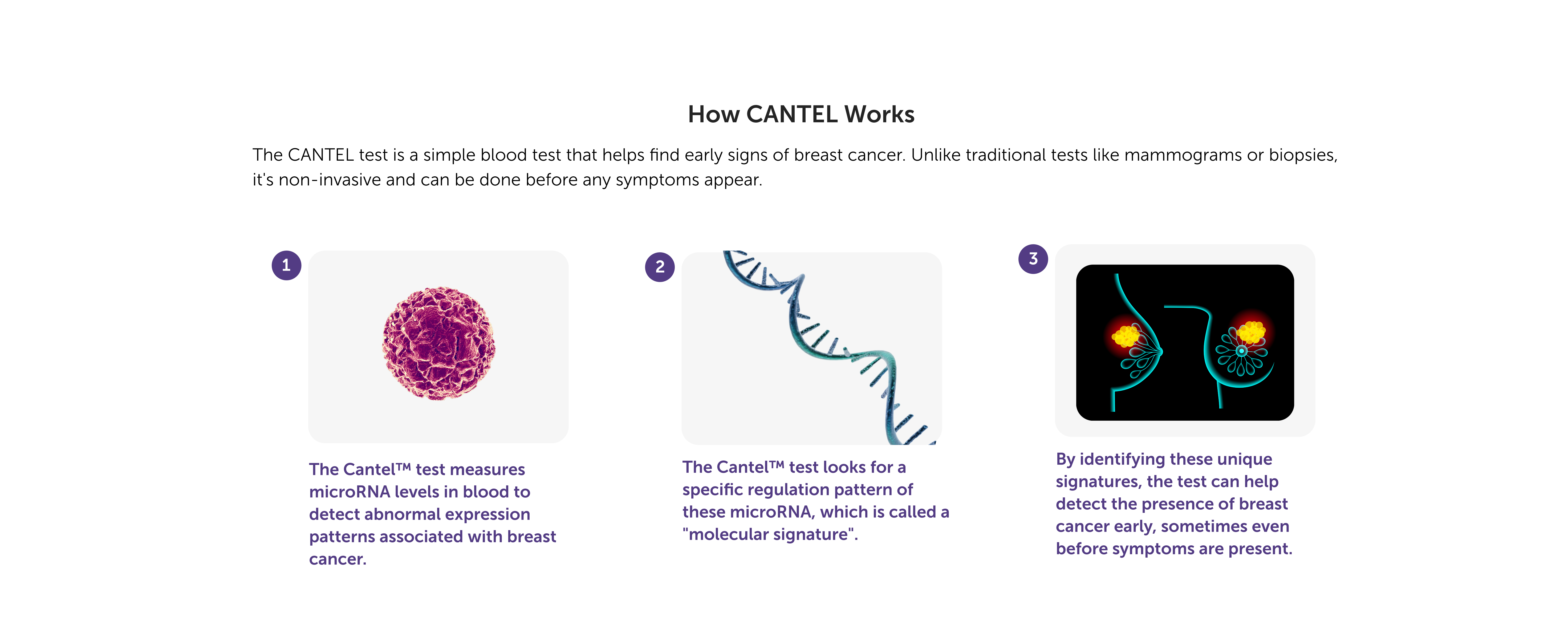
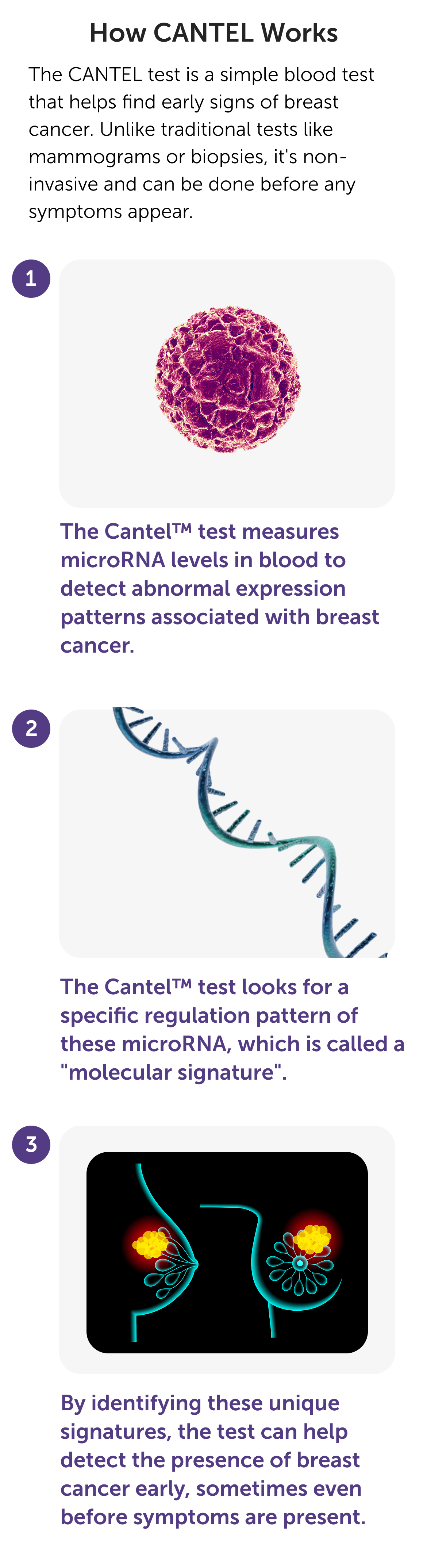
What are MicroRNAs?
MicroRNAs (miRNAs) are tiny, non-coding RNA molecules that control gene expression and serve as highly accurate, stable biomarkers found in blood. Their disease-specific profiles make them ideal for liquid biopsies, enabling early cancer detection, precision diagnostics, and personalized treatments, well before symptoms appear.

Result Interpretation
Positive result, signature detected, indicating higher likelihood of presence of breast cancer
Specific molecular signature of miRNAs was detected in the given blood sample which is suggestive of higher likelihood of presence of breast cancer. Individuals with such findings are advised consultation with their physician for appropriate guidance and additional standard of care workup as may be advised.
Note: This test may detect molecular signals associated with very early-stage breast cancer that are not yet identifiable through conventional imaging or clinical examination. These signals may reflect the earliest biological changes occurring at a subclinical stage.
Negative result, signature not detected, indicating lower likelihood of presence of breast cancer
Specific molecular signature of miRNAs was not detected in the given blood sample which is suggestive of lower likelihood of presence of breast cancer.
Note: Please be mindful that this report does not completely rule out the presence of breast cancer as molecular signatures are not present or are below detection thresholds.
Trusted Lab Network

Where your sample goes
PreRNA expert-run state-of-the-art labs for early cancer detection

Global standards
Tests adhere to stringent protocols for quality under ISO 13485 an internationally recognised quality management standard for medical devices

Certified Labs
PreRNA-approved labs with global standards for precision microRNA testing

Expert handling
Run by specialists in early cancer detection

Fully secure
End-to-end privacy and data protection
Frequently Asked Questions (FAQs)
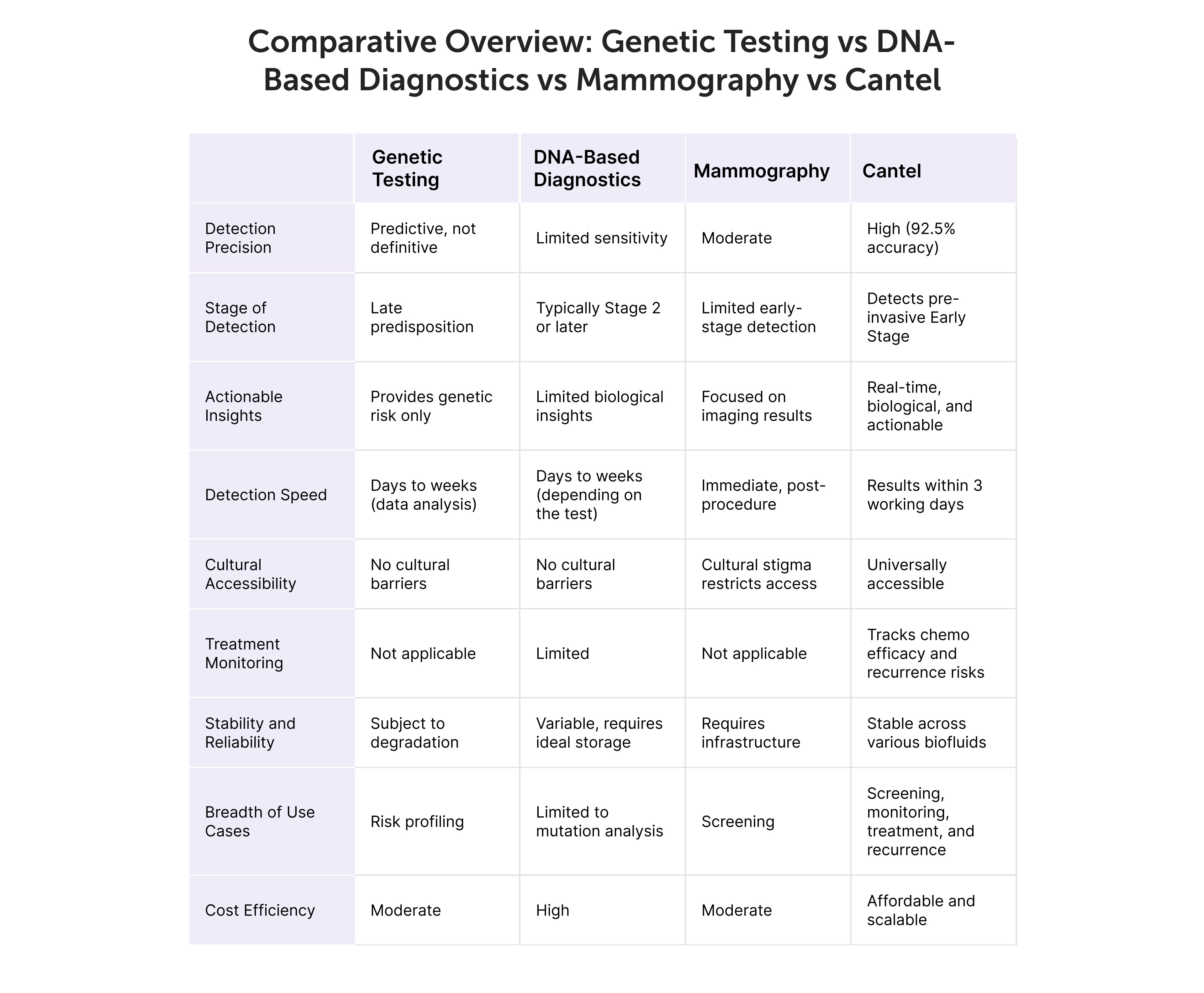
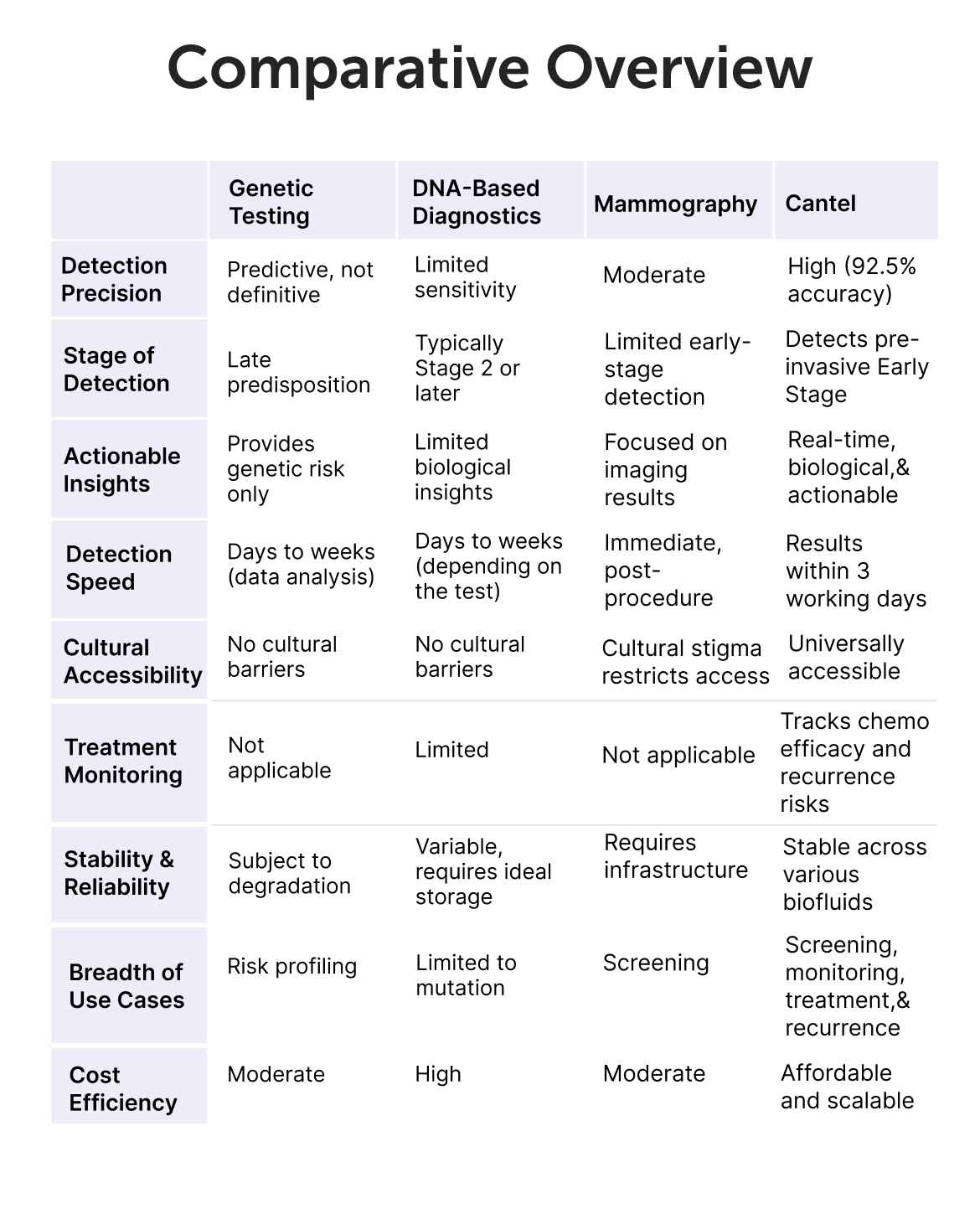
Patients with a positive result may have breast cancer should be followed by mammography. The cantel blood test can be considered in a manner similar to guideline-recommended non-invasive BC screening modalities and is not a replacement for diagnostic mammography or for surveillance mammography for high-risk individuals.
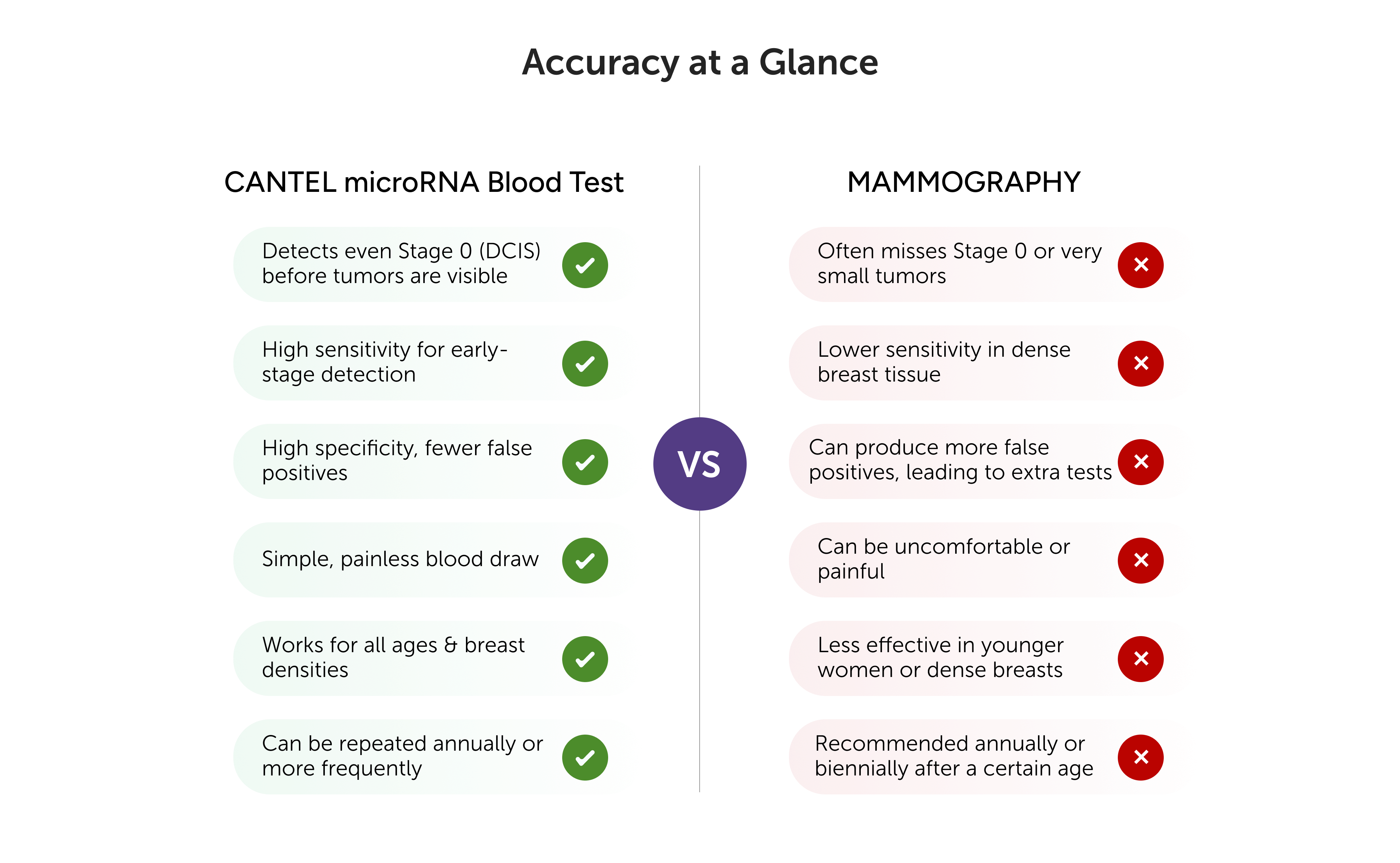
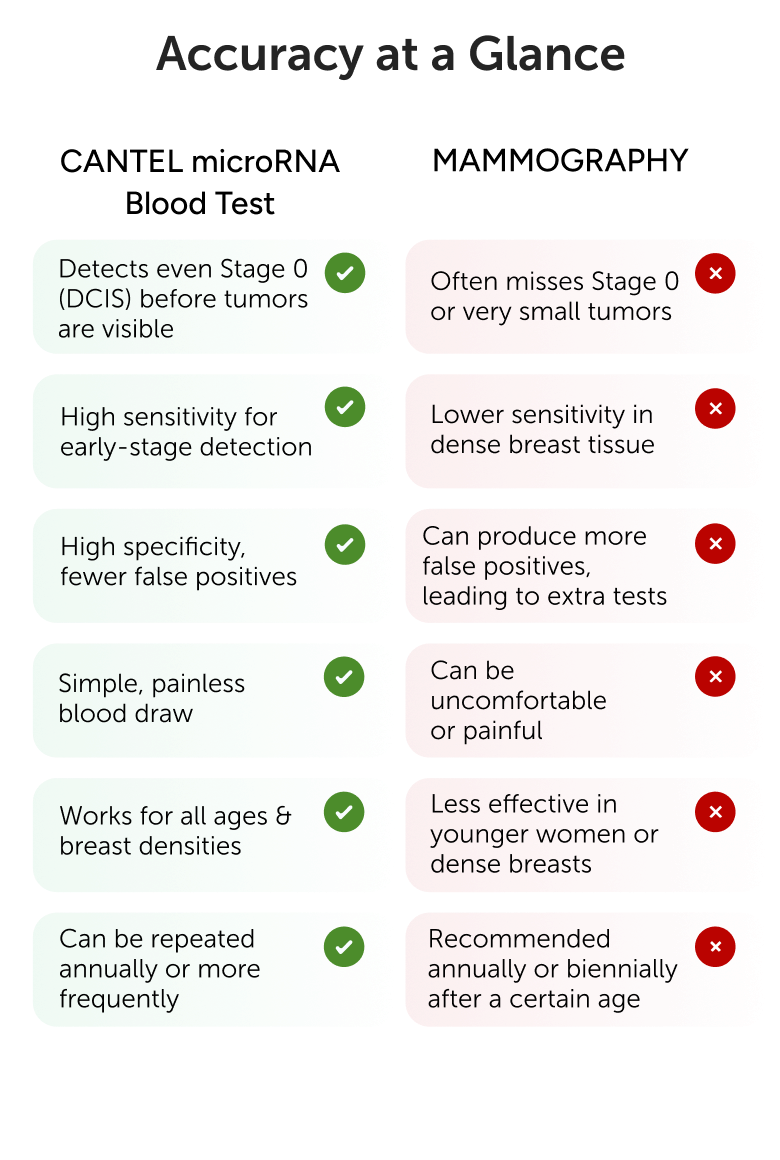
The Cantel test demonstrates an overall sensitivity of 91 % for detecting curable stage I through III Breast cancers. Specifically,

The Cantel test has been assessed by Cantel clinical study, which validated its performance as a highly sensitive blood test for BC screening, demonstrating 91% sensitivity for BC at 95% specificity.
In cases of a positive cantel test result with a negative mammography, further BC screening recommendations should be guided by the mammography findings and should continue participating in BC screening programs.
Important information to be shared with patients
The results of this test are not a guarantee of the presence or absence of breast cancer and should not be used as the sole basis for diagnosis or to replace standard medical care. False positive and false negative results may occur due to biological variations and other factors. Results should be interpreted by a qualified healthcare professional as part of a complete clinical assessment. Cantel™ test may be repeated annually. Individual is advised to consult a physician if further guidance is required.
Disclaimers
- Test results may differ from the actual state of the disease due to factors such as tumor heterogeneity, biological variations, and other influences including, but not limited to, inflammatory conditions, medication use, exposure to radiation, or other medical interventions. Additionally, errors such as sample contamination, degradation, or pre-analytical deviations may impact the accuracy of the results.
- This test is intended to assist, but not replace, the independent clinical judgment of a qualified healthcare professional. Patient care and treatment decisions should always be based on a comprehensive evaluation, including medical history, physical examination, imaging studies, histopathology, and other relevant diagnostic tests.
- False positive and false negative results are possible and should be considered when interpreting the findings. This test may detect molecular signals associated with very early-stage breast cancer that may not yet be visible through conventional imaging or clinical examination.
- The information provided by this test should be used and acted upon only by a licensed medical practitioner under applicable laws and regulations. Patients are strongly encouraged to consult with their healthcare provider to discuss the results and determine appropriate next steps.
Please read our scientific paper to learn more about the Cantel preRNA test, including full safety and validation information.